Apoptosis
What is Apoptosis?
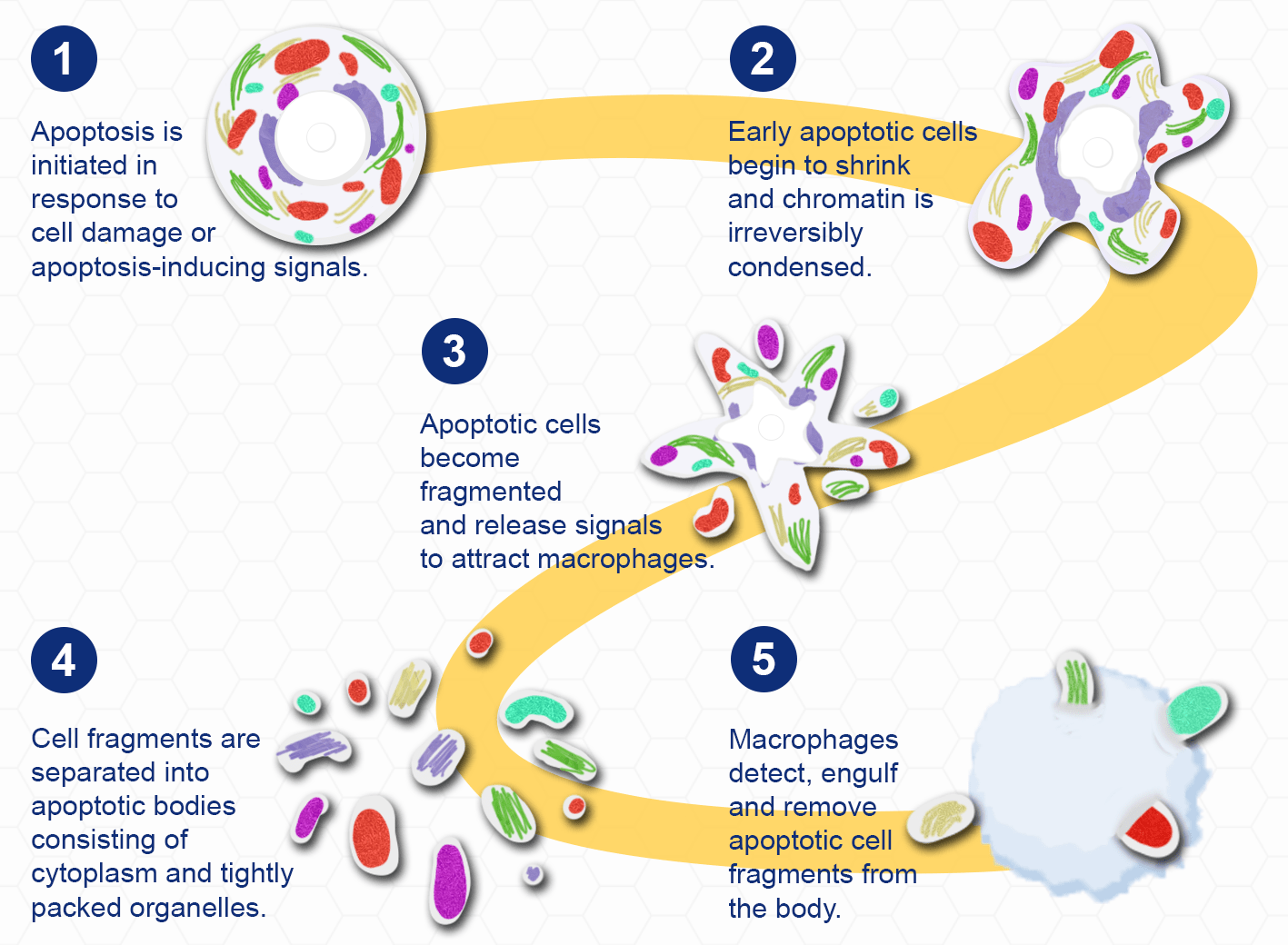
Apoptosis is a highly regulated process of programmed cell death that occurs in multicellular organisms. It primarily functions as a homeostatic mechanism to control cell populations throughout tissue development, but it also acts as a defence mechanism within the immune system and in response to cell damage. The different stages of apoptosis are characterised by distinct changes in cell morphology. During the early stages of apoptosis, cell shrinkage occurs, whereby the cells become smaller and chromatin is irreversibly condensed. This results in plasma membrane 'blebbing' and cell fragmentation. Fragments are separated into apoptotic bodies (cytoplasm, and tightly packed organelles) which are phagocytosed by macrophages and then degraded within phagolysosomes.
The mechanisms of apoptosis are highly complex, involving a cascade of energy-dependent molecular events. There are 3 main pathways which initiate apoptosis: the intrinsic pathway, the extrinsic pathway and the granzyme B pathway. These processes all result in the activation of caspase-3, and the downstream pathway which initiates the degradation of apoptotic bodies.
 |
a. Extrinsic Signalling Pathway. This pathway involves transmembrane receptor-mediated interactions. Tumour necrosis factor (TNF) receptors which, when bound by a TNF ligand (e.g. TNF-α), produce a ‘death’ signal which is transmitted from the cell surface to intracellular signalling pathways. This signal also results in the recruitment of cytoplasmic adapted proteins which associate with and activate caspase-8 via the formation of a death-inducing signalling complex (DISC). This results in the activation of the execution pathway.
b. The Intrinsic Pathway. This pathway is initiated in mitochondria via intracellular signals produced in response to cellular stress. Such signals include free radicals, toxins, radiation and the absence of certain growth factors needed for cell survival. These stimuli lead to changes in the inner mitochondrial membrane, resulting in the loss of transmembrane potential and the release of pro-apoptotic proteins from the intermembrane space into the cytoplasm. These proteins activate a caspase-dependent mitochondrial pathway, which subsequently activates caspase-9 and the execution pathway.
c. The Granzyme B Pathway. This pathway is a variant of the type IV hypersensitivity immune response, where cytotoxic T cells kill antigen-presenting cells. These cells are able to function through both the extrinsic pathway, and a novel pathway which involves the secretion of perforin and results in the formation of transmembrane pores allowing the release of ganzyme-containing cytoplasmic granules. Granzyme B the activates caspase-10 and subsequently the execution pathway.
All pathways result in the activation of the Execution Pathway. In this final pathway of apoptosis, caspase-3 is activated by caspase-8, -9, or -10. This results in the activation of CAD endonuclease, and the condensation of chromatin and cell cytoskeleton fragmentation. Cellular processes, including cell division and signal transduction, are disrupted. Caspase-3 is also involved in the ‘flipping’ of phosphatidylserine onto the surface membrane of apoptotic cells. Macrophages can then detect the externalised phosphatidylserine, engulf and destroy the apoptotic cell fragments.
 |
Studying Apoptosis
There are numerous assays that can be used to evaluate apoptosis, targeting different stages of the tightly regulated signalling cascade. Abbexa offers a wide range of apoptosis detection kits and reagents for the differentiation of live, apoptotic and dead cells using cell membrane-based and mitochondrial-based flow cytometry assays.
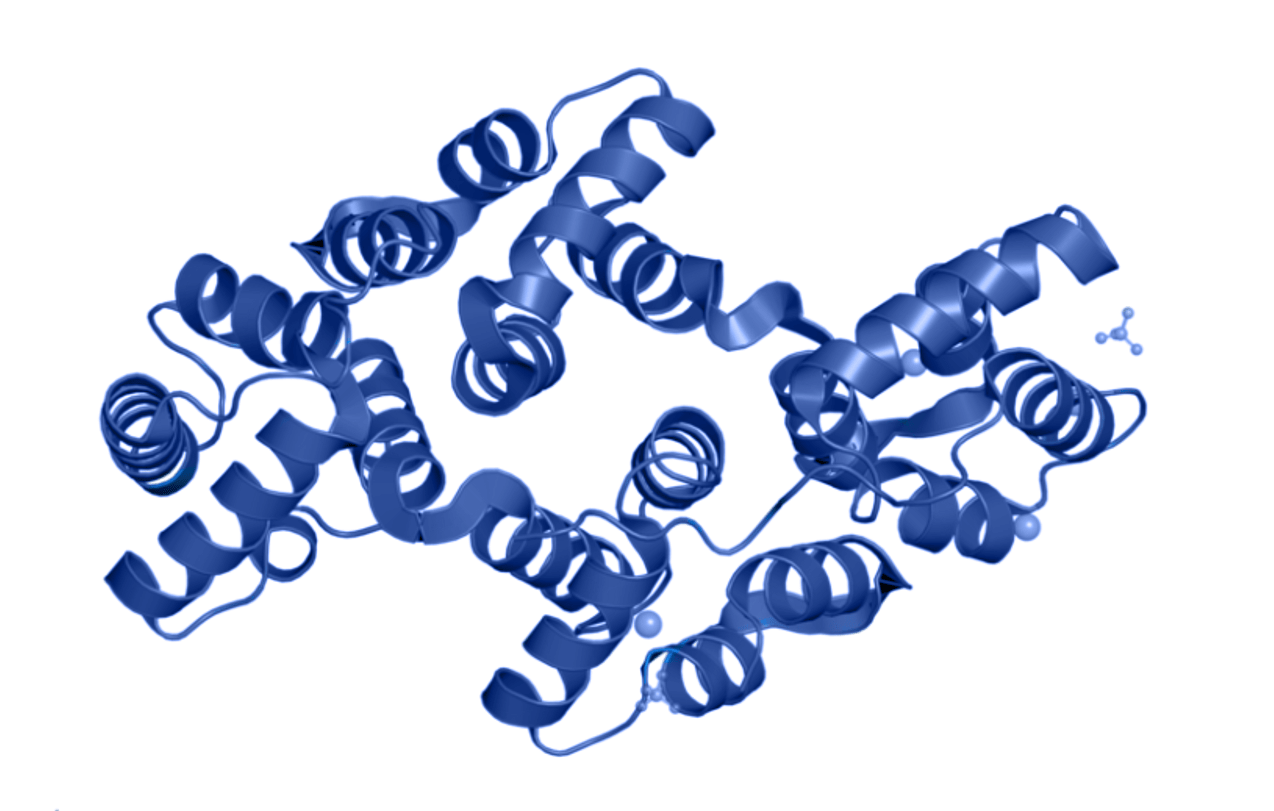
One approach for detecting and differentiating apoptotic from live cells exploits the membrane alterations which occur due to apoptosis initiation. Annexin V (blue) has a predominantly helical structure, and it binds strongly and specifically to externalised phosphatidylserine that is exclusively present on the outer plasma membrane of apoptotic cells. The protein has also been identified to bind calcium and sulfate ions (grey).
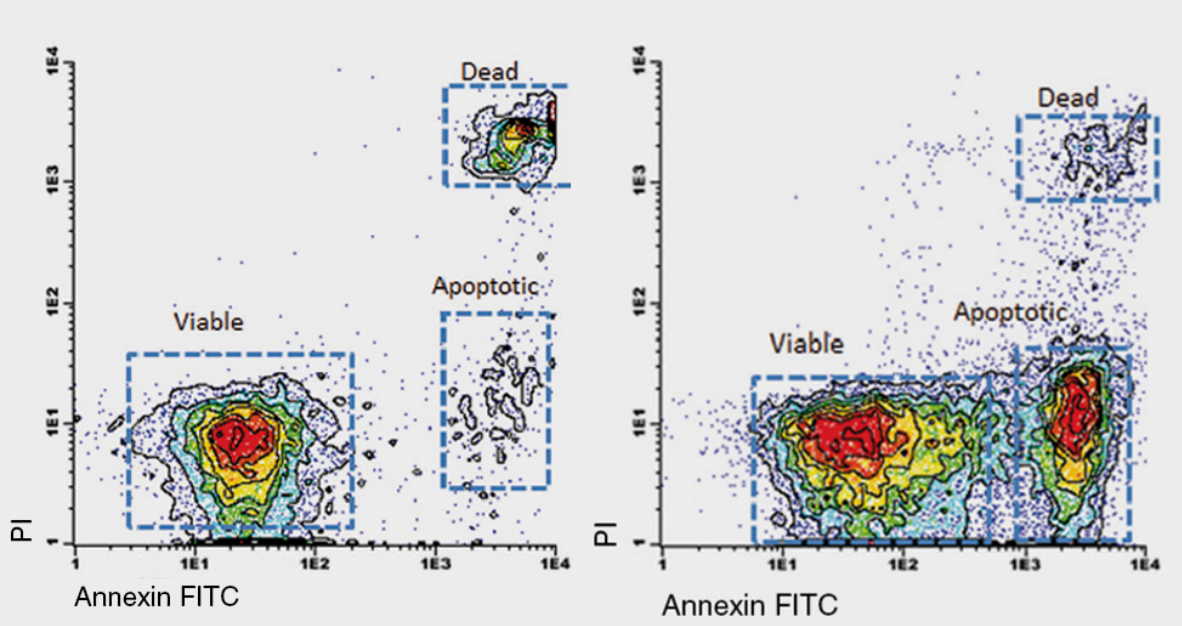
Annexin V conjugated to a fluorophore (such as Fluorescein Isothiocyanate, FITC) is frequently used in flow cytometry to allow for the fluorescent detection and sorting of individual apoptotic cells from live cells. This application is often used in conjugation with Propidium Iodide (PI) or 7-Aminoactinomycin D (7-AAD). PI and 7-AAD are DNA stains that cannot cross the plasma membrane of live cells, but can enter through membrane gaps in late apoptotic or dead cells, thus distinguishing between early apoptotic cells, and late apoptotic or dead cells. One of a the main advantages to using 7-AAD is that there is minimal spectral overlap between the emmissions of 7-AAD and the annexin V-conjugate. The result of this is the fluorescent labelling and sorting of apoptotic cells into distinct clusters when plotted on a flow cytometry dot plot.
 |
| Propidium Iodide (PI) | 7-Aminoactinomycin D (7-AAD) | |
| Chemical structure |
|
|
| Apoptosis Detection kits | Annexin V-FITC, Annexin V-APC |
Annexin V-FITC, Annexin V-PE |
| Excitation Laser Line | 488, 532, 561 (blue laser) |
488, 532, 561 (blue laser) |
| Maximum Excitation Peak | 535 |
546 |
| Maximum Emission Peak |
617 |
647 |


.png)
.png)